14 Children Dead After Consuming Contaminated Cough Syrup
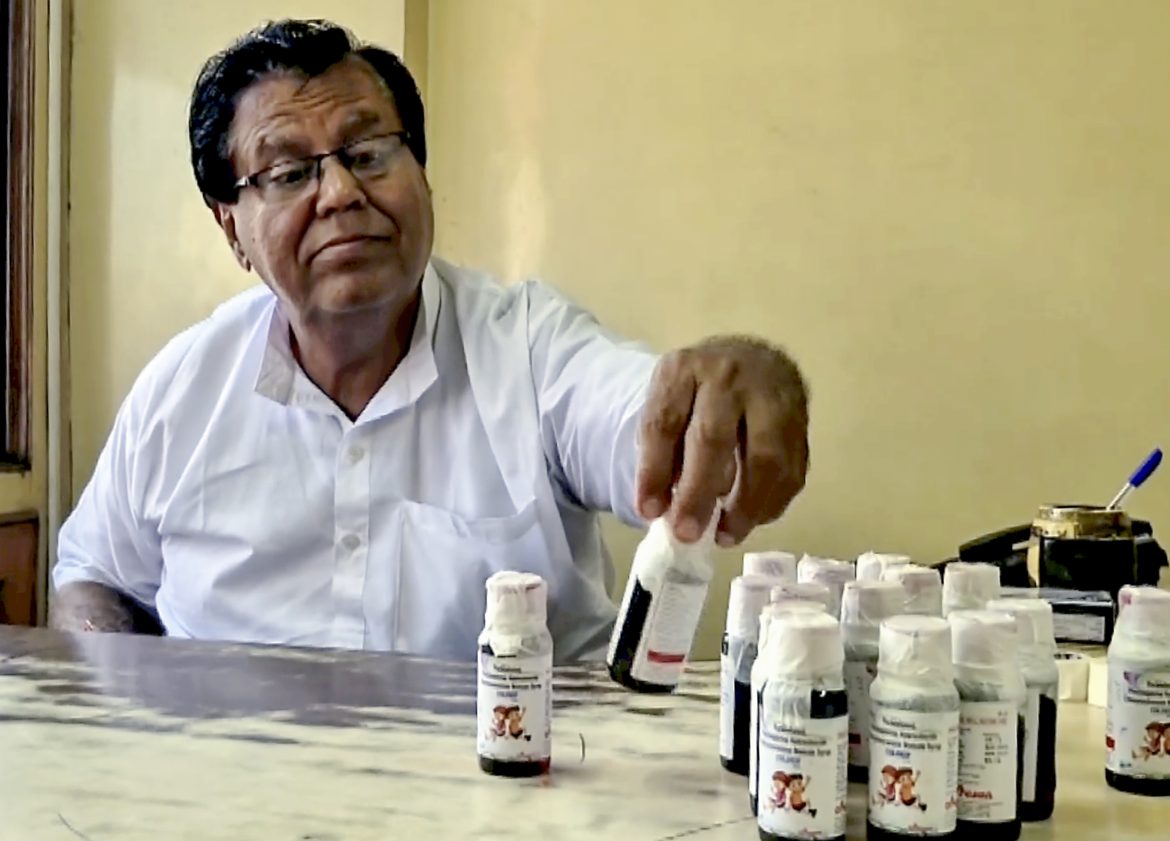
At least 14 children have died in India after allegedly consuming a contaminated cough syrup, prompting authorities in several states to impose an immediate ban on its sale and distribution. The tragic deaths have reignited concerns about drug safety and regulatory oversight in the country’s vast pharmaceutical sector.
According to Indian media reports, the fatalities were recorded across three states — Madhya Pradesh, Rajasthan, and Tamil Nadu. The worst-affected region, Madhya Pradesh, reported 11 child deaths alone, while the remaining cases were confirmed in other states.
Toxic Chemicals Found in the Syrup
Preliminary investigations have revealed that the cough syrup contained dangerously high levels of toxic chemicals, believed to have caused the children’s deaths. Local health officials have sealed medical stores and confiscated remaining stock of the product as precautionary measures.
Authorities have not yet released the exact chemical composition, but early findings suggest contamination with substances commonly associated with industrial-grade solvents, which can be lethal if ingested.
Following the discovery, several state governments swiftly banned the sale and use of the suspect syrup and ordered urgent testing of other over-the-counter medicines from the same manufacturer.
Pharmaceutical Company and Doctor Face Legal Action
Indian media reports indicate that a criminal case has been filed against the pharmaceutical company responsible for producing the tainted syrup. The firm is accused of serious violations of India’s Drugs and Cosmetics Act, including adulteration and failure to meet mandatory quality standards.
A local doctor has also been arrested for allegedly prescribing the medicine without due caution. Authorities say the doctor’s negligence may have contributed to the deaths of several children who were given the syrup to treat mild respiratory symptoms.
Law enforcement officials have sealed the company’s manufacturing unit pending a full investigation, while samples have been sent to government laboratories for forensic testing.
Public Outrage and Questions Over Drug Safety
The incident has sparked widespread outrage across India, with parents and rights groups demanding accountability from both manufacturers and regulators. Public health experts have called for a nationwide audit of pharmaceutical production facilities to prevent similar tragedies in the future.
Opposition leaders and health advocates argue that the deaths expose deep flaws in India’s drug monitoring and approval system. Many are calling for stronger enforcement of safety protocols and harsher penalties for companies found guilty of negligence.
Dr. Meena Sharma, a health policy analyst based in New Delhi, said, “This tragedy is a grim reminder of how regulatory lapses can cost innocent lives. The government must strengthen its inspection mechanisms and make quality testing mandatory before products reach pharmacies.”
History of Similar Incidents
This is not the first time India’s cough syrup exports have come under scrutiny. In 2022, at least 70 children died in The Gambia after consuming an Indian-made cough syrup later found to contain toxic chemicals such as diethylene glycol and ethylene glycol — both used in antifreeze. A year later, in 2023, a similar tragedy occurred in Uzbekistan, killing 65 children.
Those incidents prompted the World Health Organization (WHO) to issue global warnings about substandard Indian-made medicines and to call for stricter manufacturing oversight. India, which is one of the world’s largest producers of generic drugs, has since pledged to tighten safety regulations, though implementation remains inconsistent across states.
Calls for Reform and Accountability
In the wake of the latest deaths, India’s federal health ministry has directed state authorities to conduct surprise inspections of pharmaceutical plants and test batches of pediatric medicines currently in circulation. Civil society organizations are urging the government to set up a central monitoring body with real-time reporting of adverse drug reactions.
Meanwhile, families of the victims in Madhya Pradesh have demanded justice and financial compensation, saying their children died due to “systemic negligence.” Many expressed frustration that unsafe drugs continue to circulate despite previous international scandals.
A Crisis of Trust
The deaths have once again placed India’s $50-billion pharmaceutical industry under a harsh spotlight. While the country is globally recognized as the “pharmacy of the world” for its affordable generic medicines, repeated quality scandals risk undermining that reputation.
As investigations continue, public confidence in domestic drug safety remains shaken. Health experts warn that unless comprehensive reforms are introduced — including stricter licensing, random testing, and transparent reporting — tragedies like this could happen again.